Aspirin (USAN), also known as acetylsalicylic acid (INN (/əˌsɛtəlˌsælɨˈsɪlɨk/ ə-SET-əl-SAL-i-SIL-ik; ASA), is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. Aspirin was first isolated by Felix Hoffmann, a chemist with the German company Bayer in 1897.[1][2]
Salicylic acid, the main metabolite of aspirin, is an integral part of human and animal metabolism. While in humans much of it is attributable to diet, a substantial part is synthesized endogenously.[3]
Aspirin also has an antiplatelet effect by inhibiting the production of thromboxane, which under normal circumstances binds platelet molecules together to create a patch over damaged walls of blood vessels. Because the platelet patch can become too large and also block blood flow, locally and downstream, aspirin is also used long-term, at low doses, to help prevent heart attacks, strokes, and blood clot formation in people at high risk of developing blood clots.[4] It has also been established that low doses of aspirin may be given immediately after a heart attack to reduce the risk of another heart attack or of the death of cardiac tissue.[5][6] Aspirin may be effective at preventing certain types of cancer, particularly colorectal cancer.[7][8][9]
The main undesirable side effects of aspirin taken by mouth are gastrointestinal ulcers, stomach bleeding, and tinnitus, especially in higher doses. In children and adolescents, aspirin is no longer indicated to control flu-like symptoms or the symptoms of chickenpox or other viral illnesses, because of the risk of Reye’s syndrome.[10]
Aspirin is part of a group of medications called nonsteroidal anti-inflammatory drugs (NSAIDs), but differs from most other NSAIDs in the mechanism of action. Though it, and others in its group called the salicylates, have similar effects (antipyretic, anti-inflammatory, analgesic) to the other NSAIDs and inhibit the same enzyme cyclooxygenase, aspirin (but not the other salicylates) does so in an irreversible manner and, unlike others, affects more the COX-1 variant than the COX-2 variant of the enzyme.[11]
Today, aspirin is one of the most widely used medications in the world, with an estimated 40,000 tonnes of it being consumed each year.[12] In countries where Aspirin is a registered trademark owned by Bayer, the generic term is acetylsalicylic acid (ASA).[13]
Contents
1 Medical use- 1.1 Pain
- 1.2 Headache
- 1.3 Fever
- 1.4 Heart attacks and strokes
- 1.5 Post-surgery
- 1.6 Cancer prevention
- 1.7 Other uses
- 1.8 Resistance
- 1.9 Dosage
2 Adverse effects- 2.1 Contraindications
- 2.2 Gastrointestinal
- 2.3 Central effects
- 2.4 Reye’s syndrome
- 2.5 Hives and swelling
- 2.6 Other adverse effects
- 2.7 Overdose
- 2.8 Interactions
- 3 Chemical properties
4 Physical properties
5 Mechanism of action- 5.1 Discovery of the mechanism
- 5.2 Suppression of prostaglandins and thromboxanes
- 5.3 COX-1 and COX-2 inhibition
- 5.4 Additional mechanisms
- 5.5 Hypothalamic-pituitary-adrenal activity
- 6 Pharmacokinetics
7 History- 8 Compendial status
- 9 Veterinary use
10 References- 11 External links
Medical use
Aspirin is used in the treatment of a number of conditions, including fever, pain, rheumatic fever, and inflammatory diseases, such as rheumatoid arthritis, pericarditis, and Kawasaki disease.[14] Lower doses of aspirin have also shown to reduce the risk of death from a heart attack, or the risk of stroke in some circumstances.[15][16][17] There is some evidence that aspirin is effective at preventing colorectal cancer, though the mechanisms of this effect are unclear.[18]
Pain

In most cases, aspirin is considered inferior to ibuprofen for the alleviation of pain, because aspirin is more likely to cause gastrointestinal bleeding.[19] Aspirin is generally ineffective for those pains caused by muscle cramps, bloating, gastric distension, or acute skin irritation.[20] As with other NSAIDs, combinations of aspirin and caffeine provide slightly greater pain relief than aspirin alone.[21]Effervescent formulations of aspirin, such as Alka-Seltzer or Blowfish,[22] relieve pain faster than aspirin in tablets,[23] which makes them useful for the treatment of migraines.[24]
Topical aspirin may be effective for treating some types of neuropathic pain.[25]
Headache
Aspirin, either by itself or in combined formulation, effectively treats some types of headache, but its efficacy may be questionable for others. Secondary headaches, meaning those caused by another disorder or trauma, should be promptly treated by a medical provider.
Among primary headaches, the International Classification of Headache Disorders distinguishes between tension headache (the most common), migraine, and cluster headache. Aspirin or other over-the-counter analgesics are widely recognized as effective for the treatment of tension headache.[26] Aspirin, especially as a component of an acetaminophen/aspirin/caffeine formulation, e.g., Excedrin Migraine, is considered a first-line therapy in the treatment of migraine, and comparable to lower doses of sumatriptan. It is most effective at stopping migraines when they are first beginning.[27] There is little data that suggest the aspirin is an effective treatment for cluster headache.
Fever
Like its ability to control pain, aspirin’s ability to control fever is due to its action on the prostaglandin system through its irreversible inhibition of COX.[28] Although aspirin’s use as an antipyretic in adults is well-established, many medical societies and regulatory agencies (including the American Academy of Family Physicians, the American Academy of Pediatrics, and the U.S. Food and Drug Administration (FDA)) strongly advise against using aspirin for treatment of fever in children because of the risk of Reye’s syndrome, a rare but often fatal illness associated with the use of aspirin or other salicylates in children during episodes of viral or bacterial infection.[29][30][31] Because of the risk of Reye’s syndrome in children, in 1986, the FDA required labeling on all aspirin-containing medications advising against its use in children and teenagers.[32]
Heart attacks and strokes
For a subset of the population, aspirin may help prevent heart attacks and strokes. In lower doses, aspirin has been known to prevent the progression of existing cardiovascular disease, and reduce the frequency of these events for those with a history of them.[33][34] (This is known as secondary prevention.)
Aspirin appears to offer little benefit to those at lower risk of heart attack or stroke—for instance, those without a history of these events or with pre-existing disease. (This is called primary prevention.) Some studies recommend aspirin on a case-by-case basis,[35][36] while others have suggested that the risks of other events, such as gastrointestinal bleeding, were significant enough to outweigh any potential benefit, and recommended against using aspirin for primary prevention entirely.[37]
Complicating the use of aspirin for prevention is the phenomenon of aspirin resistance.[38][39] For patients who are resistant, aspirin’s efficacy is reduced, which can cause an increased risk of stroke.[40] Some authors have suggested testing regimes to identify those patients who are resistant to aspirin or other anti-thrombotic drugs (such as clopidogrel).[41]
Aspirin has also been suggested as a component of a polypill[42][43] for prevention of cardiovascular disease.
Post-surgery
After percutaneous coronary interventions (PCIs), such as the placement of a coronary artery stent, a U.S. Agency for Healthcare Research and Quality guideline recommends that aspirin be taken indefinitely.[44] Frequently, aspirin is combined with an ADP receptor inhibitor, such as clopidogrel, prasugrel or ticagrelor to prevent blood clots. This is called dual anti-platelet therapy (DAPT). United States and European Union guidelines disagree somewhat about how long, and for what indications this combined therapy should be continued post-surgery. U.S. guidelines recommend DAPT for at least 12 months while EU guidelines recommend DAPT for 6–12 months after drug eluting stent.[45] However, they agree that aspirin be continued indefinitely after DAPT is complete.
Cancer prevention
Aspirin’s effect on cancer has been widely studied, particularly its effect on colorectal cancer (CRC). Multiple meta-analyses and reviews have concluded that regular use of aspirin reduces the long-term risk of CRC incidence and mortality.[18][46][47][48] However, the relationships of aspirin dose and duration of use to the various types of CRC risk, including mortality, progression, and incidence, are not well-defined. While the majority of data on aspirin and CRC risk comes from observational studies, rather than randomized controlled trials (RCTs), the available data from RCTs suggests that long-term use of low dose aspirin may be effective at preventing some types of CRC.[49] In the 2007 United States Preventive Services Task Force (USPSTF) guidelines on this topic, use of aspirin for prevention of CRC was given a “D” rating,[50] advising healthcare practitioners against routinely using aspirin for this purpose.
Other uses
Aspirin is a first-line treatment for the fever and joint pain symptoms of acute rheumatic fever. The therapy often lasts for one to two weeks, and is rarely indicated for longer periods. After fever and pain have subsided, the aspirin is no longer necessary, since it does not decrease the incidence of heart complications and residual rheumatic heart disease.[51][52]Naproxen has been shown to be as effective as aspirin and less toxic, but due to the limited clinical experience, naproxen is recommended only as a second-line treatment.[51][53]
Along with rheumatic fever, Kawasaki disease remains one of the few indications for aspirin use in children[54] in spite of a lack of high quality evidence for its effectiveness.[55]
Low dose aspirin supplementation has moderate benefits when used for prevention of pre-eclampsia.[56][57]
Resistance
For some people, aspirin does not have as strong an effect on platelets as for others, an effect known as aspirin resistance or insensitivity. One study has suggested women are more likely to be resistant than men,[58] and a different, aggregate study of 2,930 patients found 28% were resistant.[59] A study in 100 Italian patients, on the other hand, found that, of the apparent 31% aspirin-resistant subjects, only 5% were truly resistant, and the others were noncompliant.[60] Another study of 400 healthy volunteers found no subjects who were truly resistant, but some had “pseudoresistance, reflecting delayed and reduced drug absorption.”[61]
Dosage
Adult aspirin tablets are produced in standardised sizes, which vary slightly from country to country, for example 300Â mg in Britain and 325Â mg in the United States. Smaller doses are based on these standards, e.g., 75Â mg and 81Â mg tablets. The 81Â mg tablets are called “baby-strength”, even though they are not intended to be administered to infants and children. There is no medical significance in the slight difference in dosage between the 75Â mg and the 81Â mg tablets. Of historic interest, in the United States, a 325Â mg dose is equivalent to the historic 5 grain aspirin tablet in use prior to the metric system.
In general, for adults, doses are taken four times a day for fever or arthritis,[62] with doses near the maximal daily dose used historically for the treatment of rheumatic fever.[63] For the prevention of myocardial infarction in someone with documented or suspected coronary artery disease, much lower doses are taken once daily.[62]
Recommendations from the USPSTF[64] on the use of aspirin for the primary prevention of coronary heart disease encourage men aged 45–79 and women aged 55–79 to use aspirin when the potential benefit of a reduction in myocardial infarction (MI) for men or stroke for women outweighs the potential harm of an increase in gastrointestinal hemorrhage.[65] The WHI study said regular low dose (75 or 81 mg) aspirin female users had a 25% lower risk of death from cardiovascular disease and a 14% lower risk of death from any cause.[65] Low dose aspirin use was also associated with a trend toward lower risk of cardiovascular events, and lower aspirin doses (75 or 81 mg/day) may optimize efficacy and safety for patients requiring aspirin for long-term prevention.[65]
In children with Kawasaki disease, aspirin is taken at dosages based on body weight, initially four times a day for up to two weeks and then at a lower dose once daily for a further six to eight weeks.[66]
Adverse effects
Contraindications
Aspirin should not be taken by people who are allergic to ibuprofen or naproxen,[67][68] or who have salicylate intolerance[69][70] or a more generalized drug intolerance to NSAIDs, and caution should be exercised in those with asthma or NSAID-precipitated bronchospasm. Owing to its effect on the stomach lining, manufacturers recommend people with peptic ulcers, mild diabetes, or gastritis seek medical advice before using aspirin.[67][71] Even if none of these conditions is present, the risk of stomach bleeding is still increased when aspirin is taken with alcohol or warfarin.[67][68] Patients with hemophilia or other bleeding tendencies should not take aspirin or other salicylates.[67][71] Aspirin is known to cause hemolytic anemia in people who have the genetic disease glucose-6-phosphate dehydrogenase deficiency, particularly in large doses and depending on the severity of the disease.[72] Use of aspirin during dengue fever is not recommended owing to increased bleeding tendency.[73] People with kidney disease, hyperuricemia, or gout should not take aspirin because it inhibits the kidneys’ ability to excrete uric acid, and thus may exacerbate these conditions. Aspirin should not be given to children or adolescents to control cold or influenza symptoms, as this has been linked with Reye’s syndrome.[10]
Gastrointestinal
Aspirin use has been shown to increase the risk of gastrointestinal bleeding.[74] Although some enteric-coated formulations of aspirin are advertised as being “gentle to the stomach”, in one study, enteric coating did not seem to reduce this risk.[74] Combining aspirin with other NSAIDs has also been shown to further increase this risk.[74] Using aspirin in combination with clopidogrel or warfarin also increases the risk of upper gastrointestinal bleeding.[75]
It appears that blockade of COX-1 by aspirin results in the upregulation of COX-2 as part of a gastric defense[76] and that taking COX-2 inhibitors concurrently with aspirin increases the gastric mucosal erosion.[77] Therefore, caution should be exercised if combining aspirin with any “natural” supplements with COX-2 inhibiting properties, such as garlic extracts, curcumin, bilberry, pine bark, ginkgo, fish oil, resveratrol, genistein, quercetin, resorcinol, and others.
In addition to enteric coating, “buffering” is the other main method companies have used to try to mitigate the problem of gastrointestinal bleeding. Buffering agents are intended to work by preventing the aspirin from concentrating in the walls of the stomach, although the benefits of buffered aspirin are disputed. Almost any buffering agent used in antacids can be used; Bufferin, for example, uses MgO. Other preparations use CaCO3.[78]
Taking it with vitamin C is a more recently investigated method of protecting the stomach lining. Taking equal doses of vitamin C and aspirin may decrease the amount of stomach damage that occurs compared to taking aspirin alone.[79][80]
Central effects
Large doses of salicylate, a metabolite of aspirin, have been proposed to cause tinnitus (ringing in the ears) based on experiments in rats, via the action on arachidonic acid and NMDA receptors cascade.[81]
Reye’s syndrome
Reye’s syndrome, a rare but severe illness characterized by acute encephalopathy and fatty liver, can occur when children or adolescents are given aspirin for a fever or other illnesses or infections. From 1981 through 1997, 1207 cases of Reye’s syndrome in under-18 patients were reported to the U.S. Centers for Disease Control and Prevention. Of these, 93% reported being ill in the three weeks preceding onset of Reye’s syndrome, most commonly with a respiratory infection, chickenpox, or diarrhea. Salicylates were detectable in 81.9% of children for whom test results were reported.[82] After the association between Reye’s syndrome and aspirin was reported, and safety measures to prevent it (including a Surgeon General‘s warning, and changes to the labeling of aspirin-containing drugs) were implemented, aspirin taken by children declined considerably in the United States, as did the number of reported cases of Reye’s syndrome; a similar decline was found in the United Kingdom after warnings against pediatric aspirin use were issued.[82] The U.S. Food and Drug Administration now recommends aspirin (or aspirin-containing products) should not be given to anyone under the age of 12 who has a fever,[10] and the British Medicines and Healthcare products Regulatory Agency recommends children who are under 16 years of age should not take aspirin, unless it is on the advice of a doctor.[83]
Hives and swelling
For a small number of people, taking aspirin can result in symptoms resembling an allergic reaction, including hives, swelling and headache. The reaction is caused by salicylate intolerance and is not a true allergy, but rather an inability to metabolize even small amounts of aspirin, resulting in an overdose.
Other adverse effects
Aspirin can induce angioedema (swelling of skin tissues) in some people. In one study, angioedema appeared one to six hours after ingesting aspirin in some of the patients. However, when the aspirin was taken alone, it did not cause angioedema in these patients; the aspirin had been taken in combination with another NSAID-induced drug when angioedema appeared.[84]
Aspirin causes an increased risk of cerebral microbleeds having the appearance on MRI scans of 5 to 10Â mm or smaller, hypointense (dark holes) patches.[85][86] Such cerebral microbleeds are important, since they often occur prior to ischemic stroke or intracerebral hemorrhage, Binswanger disease and Alzheimer’s disease.
A study of a group with a mean dosage of aspirin of 270Â mg per day estimated an average absolute risk increase in intracerebral hemorrhage (ICH) of 12 events per 10,000 persons.[87] In comparison, the estimated absolute risk reduction in myocardial infarction was 137 events per 10,000 persons, and a reduction of 39 events per 10,000 persons in ischemic stroke.[87] In cases where ICH already has occurred, aspirin use results in higher mortality, with a dose of approximately 250Â mg per day resulting in a relative risk of death within three months after the ICH of approximately 2.5 (95% confidence interval 1.3 to 4.6).[88]
Aspirin and other NSAIDs can cause hyperkalemia by inducing a hyporenin hypoaldosteronic state via inhibition of prostaglandin synthesis; however, these agents do not typically cause hyperkalemia by themselves in the setting of normal renal function and euvolemic state.[89]
Aspirin can cause prolonged bleeding after operations for up to 10 days. In one study, 30 of 6499 elective surgical patients required reoperations to control bleeding. Twenty had diffuse bleeding and 10 had bleeding from a site. Diffuse, but not discrete, bleeding was associated with the preoperative use of aspirin alone or in combination with other NSAIDS in 19 of the 20 diffuse bleeding patients.[90]
| Condition | Prothrombin time | Partial thromboplastin time | Bleeding time | Platelet count |
|---|---|---|---|---|
Vitamin K deficiency or warfarin | Prolonged | Normal or mildly prolonged | Unaffected | Unaffected |
| Disseminated intravascular coagulation | Prolonged | Prolonged | Prolonged | Decreased |
| Von Willebrand disease | Unaffected | Prolonged or unaffected | Prolonged | Unaffected |
| Hemophilia | Unaffected | Prolonged | Unaffected | Unaffected |
| Aspirin | Unaffected | Unaffected | Prolonged | Unaffected |
| Thrombocytopenia | Unaffected | Unaffected | Prolonged | Decreased |
Liver failure, early | Prolonged | Unaffected | Unaffected | Unaffected |
| Liver failure, end-stage | Prolonged | Prolonged | Prolonged | Decreased |
| Uremia | Unaffected | Unaffected | Prolonged | Unaffected |
| Congenital afibrinogenemia | Prolonged | Prolonged | Prolonged | Unaffected |
Factor V deficiency | Prolonged | Prolonged | Unaffected | Unaffected |
Factor X deficiency as seen in amyloid purpura | Prolonged | Prolonged | Unaffected | Unaffected |
| Glanzmann’s thrombasthenia | Unaffected | Unaffected | Prolonged | Unaffected |
| Bernard-Soulier syndrome | Unaffected | Unaffected | Prolonged | Decreased or unaffected |
| Factor XII deficiency | Unaffected | Prolonged | Unaffected | Unaffected |
| C1INH deficiency | Unaffected | Shortened | Unaffected | Unaffected |
Overdose
Aspirin overdose can be acute or chronic. In acute poisoning, a single large dose is taken; in chronic poisoning, higher than normal doses are taken over a period of time. Acute overdose has a mortality rate of 2%. Chronic overdose is more commonly lethal, with a mortality rate of 25%;[91] chronic overdose may be especially severe in children.[92] Toxicity is managed with a number of potential treatments, including activated charcoal, intravenous dextrose and normal saline, sodium bicarbonate, and dialysis.[93] The diagnosis of poisoning usually involves measurement of plasma salicylate, the active metabolite of aspirin, by automated spectrophotometric methods. Plasma salicylate levels in general range from 30–100 mg/l after usual therapeutic doses, 50–300 mg/l in patients taking high doses and 700–1400 mg/l following acute overdose. Salicylate is also produced as a result of exposure to bismuth subsalicylate, methyl salicylate and sodium salicylate.[94][95]
Interactions
Aspirin is known to interact with other drugs. For example, acetazolamide and ammonium chloride are known to enhance the intoxicating effect of salicyclates, and alcohol also increases the gastrointestinal bleeding associated with these types of drugs.[67][68] Aspirin is known to displace a number of drugs from protein-binding sites in the blood, including the antidiabetic drugs tolbutamide and chlorpropamide, the immunosuppressant methotrexate, phenytoin, probenecid, valproic acid (as well as interfering with beta oxidation, an important part of valproate metabolism) and any NSAID. Corticosteroids may also reduce the concentration of aspirin. Ibuprofen can negate the antiplatelet effect of aspirin used for cardioprotection and stroke prevention.[96] The pharmacological activity of spironolactone may be reduced by taking aspirin, and aspirin is known to compete with penicillin G for renal tubular secretion.[97] Aspirin may also inhibit the absorption of vitamin C.[98][99][100]
Chemical properties
Acetylsalicylic acid (ASA) decomposes rapidly in solutions of ammonium acetate or of the acetates, carbonates, citrates or hydroxides of the alkali metals. ASA is stable in dry air, but gradually hydrolyses in contact with moisture to acetic and salicylic acids. In solution with alkalis, the hydrolysis proceeds rapidly and the clear solutions formed may consist entirely of acetate and salicylate.[101]
Physical properties
Aspirin, an acetyl derivative of salicylic acid, is a white, crystalline, weakly acidic substance, with a melting point of 136 °C (277 °F), and a boiling point of 140 °C (284 °F).[102]
Synthesis
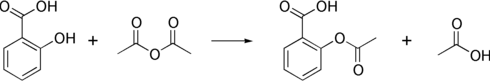
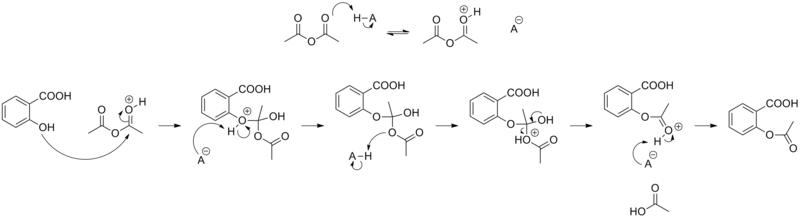
The synthesis of aspirin is classified as an esterification reaction. Salicylic acid is treated with acetic anhydride, an acid derivative, causing a chemical reaction that turns salicylic acid’s hydroxyl group into an ester group (R-OH → R-OCOCH3). This process yields aspirin and acetic acid, which is considered a byproduct of this reaction. Small amounts of sulfuric acid (and occasionally phosphoric acid) are almost always used as a catalyst. This method is commonly employed in undergraduate teaching labs.[103]
Formulations containing high concentrations of aspirin often smell like vinegar[104] because aspirin can decompose through hydrolysis in moist conditions, yielding salicylic and acetic acids.[105]
The acid dissociation constant (pKa) for acetylsalicylic acid is 3.5 at 25°.[106]
Polymorphism
Polymorphism, or the ability of a substance to form more than one crystal structure, is important in the development of pharmaceutical ingredients. Many drugs are receiving regulatory approval for only a single crystal form or polymorph. For a long time, only one crystal structure for aspirin was known. That aspirin might have a second crystalline form was suspected since the 1960s. The elusive second polymorph was first discovered by Vishweshwar and coworkers in 2005,[107] and fine structural details were given by Bond et al.[108] A new crystal type was found after attempted cocrystallization of aspirin and levetiracetam from hot acetonitrile. The form II is only stable at 100 K and reverts to form I at ambient temperature. In the (unambiguous) form I, two salicylic molecules form centrosymmetric dimers through the acetyl groups with the (acidic) methyl proton to carbonyl hydrogen bonds, and in the newly claimed form II, each salicylic molecule forms the same hydrogen bonds with two neighboring molecules instead of one. With respect to the hydrogen bonds formed by the carboxylic acid groups, both polymorphs form identical dimer structures.
Mechanism of action
Discovery of the mechanism
In 1971, British pharmacologist John Robert Vane, then employed by the Royal College of Surgeons in London, showed aspirin suppressed the production of prostaglandins and thromboxanes.[109][110] For this discovery he was awarded the 1982 Nobel Prize in Physiology or Medicine, jointly with Sune K. Bergström and Bengt I. Samuelsson.[111] In 1984 he was made a Knight Bachelor.
Suppression of prostaglandins and thromboxanes
Aspirin’s ability to suppress the production of prostaglandins and thromboxanes is due to its irreversible inactivation of the cyclooxygenase (PTGS) enzyme required for prostaglandin and thromboxane synthesis. Aspirin acts as an acetylating agent where an acetyl group is covalently attached to a serine residue in the active site of the PTGS enzyme. This makes aspirin different from other NSAIDs (such as diclofenac and ibuprofen), which are reversible inhibitors.
Low-dose, long-term aspirin use irreversibly blocks the formation of thromboxane A2 in platelets, producing an inhibitory effect on platelet aggregation. This antithrombotic property makes aspirin useful for reducing the incidence of heart attacks.[112] 40Â mg of aspirin a day is able to inhibit a large proportion of maximum thromboxane A2 release provoked acutely, with the prostaglandin I2 synthesis being little affected; however, higher doses of aspirin are required to attain further inhibition.[113]
Prostaglandins, local hormones produced in the body, have diverse effects, including the transmission of pain information to the brain, modulation of the hypothalamic thermostat, and inflammation. Thromboxanes are responsible for the aggregation of platelets that form blood clots. Heart attacks are caused primarily by blood clots, and low doses of aspirin are seen as an effective medical intervention for acute myocardial infarction. An unwanted side effect of the effective anticlotting action of aspirin is that it may cause excessive bleeding.
COX-1 and COX-2 inhibition
There are at least two different types of cyclooxygenase: COX-1 and COX-2. Aspirin irreversibly inhibits COX-1 and modifies the enzymatic activity of COX-2. COX-2 normally produces prostanoids, most of which are proinflammatory. Aspirin-modified PTGS2 produces lipoxins, most of which are anti-inflammatory.[114] Newer NSAID drugs, COX-2 inhibitors (coxibs), have been developed to inhibit only PTGS2, with the intent to reduce the incidence of gastrointestinal side effects.[12]
However, several of the new COX-2 inhibitors, such as rofecoxib (Vioxx), have been withdrawn recently, after evidence emerged that PTGS2 inhibitors increase the risk of heart attack and stroke.[115][116] Endothelial cells lining the microvasculature in the body are proposed to express PTGS2, and, by selectively inhibiting PTGS2, prostaglandin production (specifically, PGI2; prostacyclin) is downregulated with respect to thromboxane levels, as PTGS1 in platelets is unaffected. Thus, the protective anticoagulative effect of PGI2 is removed, increasing the risk of thrombus and associated heart attacks and other circulatory problems. Since platelets have no DNA, they are unable to synthesize new PTGS once aspirin has irreversibly inhibited the enzyme, an important difference with reversible inhibitors.
Additional mechanisms
Aspirin has been shown to have at least three additional modes of action. It uncouples oxidative phosphorylation in cartilaginous (and hepatic) mitochondria, by diffusing from the inner membrane space as a proton carrier back into the mitochondrial matrix, where it ionizes once again to release protons.[117] In short, aspirin buffers and transports the protons. When high doses of aspirin are given, it may actually cause fever, owing to the heat released from the electron transport chain, as opposed to the antipyretic action of aspirin seen with lower doses. In addition, aspirin induces the formation of NO-radicals in the body, which have been shown in mice to have an independent mechanism of reducing inflammation. This reduced leukocyte adhesion, which is an important step in immune response to infection; however, there is currently insufficient evidence to show that aspirin helps to fight infection.[118] More recent data also suggest salicylic acid and its derivatives modulate signaling through NF-κB.[119] NF-κB, a transcription factor complex, plays a central role in many biological processes, including inflammation.
Aspirin is readily broken down in the body to salicylic acid, which itself has anti-inflammatory, antipyretic, and analgesic effects. In 2012, salicylic acid was found to activate AMP-activated protein kinase, and this has been suggested as a possible explanation for some of the effects of both salicylic acid and aspirin.[120][121] The acetyl portion of the aspirin molecule is not without its own targets. Acetylation of cellular proteins is a well-established phenomenon in the regulation of protein function at the posttranslational level. Recent studies have reported aspirin is able to acetylate several other targets in addition to COX isoenzymes.[122][123] These acetylation reactions may explain many hitherto unexplained effects of aspirin.
Hypothalamic-pituitary-adrenal activity
Aspirin, like other medications affecting prostaglandin synthesis, has profound effects on the pituitary gland, which indirectly affects a number of other hormones and physiological functions. Effects on growth hormone, prolactin,[124] and TSH (with relevant effect on T3 and T4) were observed directly.[125] Aspirin reduces the effects of vasopressin[126] and increases those of naloxone[127] upon the secretion of ACTH and cortisol by the hypothalamic-pituitary-adrenal axis (HPA axis), which has been suggested to occur through an interaction with endogenous prostaglandins and their role in regulating the HPA axis.[126]
Pharmacokinetics
Salicylic acid is a weak acid, and very little of it is ionized in the stomach after oral administration. Acetylsalicylic acid is poorly soluble in the acidic conditions of the stomach, which can delay absorption of high doses for eight to 24 hours. The increased pH and larger surface area of the small intestine causes aspirin to be absorbed rapidly there, which in turn allows more of the salicylate to dissolve. Owing to the issue of solubility, however, aspirin is absorbed much more slowly during overdose, and plasma concentrations can continue to rise for up to 24 hours after ingestion.[128][129][130]
About 50–80% of salicylate in the blood is bound to albumin protein, while the rest remains in the active, ionized state; protein binding is concentration-dependent. Saturation of binding sites leads to more free salicylate and increased toxicity. The volume of distribution is 0.1–0.2 l/kg. Acidosis increases the volume of distribution because of enhancement of tissue penetration of salicylates.[130]
As much as 80% of therapeutic doses of salicylic acid is metabolized in the liver. Conjugation with glycine forms salicyluric acid, and with glucuronic acid it forms salicyl acyl and phenolic glucuronide. These metabolic pathways have only a limited capacity. Small amounts of salicylic acid are also hydroxylated to gentisic acid. With large salicylate doses, the kinetics switch from first order to zero order, as metabolic pathways become saturated and renal excretion becomes increasingly important.[130]
Salicylates are excreted mainly by the kidneys as salicyluric acid (75%), free salicylic acid (10%), salicylic phenol (10%), and acyl glucuronides (5%), gentisic acid (< 1%), and 2,3-dihydroxybenzoic acid.[131] When small doses (less than 250 mg in an adult) are ingested, all pathways proceed by first-order kinetics, with an elimination half-life of about 2.0 to 4.5 hours.[132][133] When higher doses of salicylate are ingested (more than 4 g), the half-life becomes much longer (15–30 hours),[134] because the biotransformation pathways concerned with the formation of salicyluric acid and salicyl phenolic glucuronide become saturated.[135] Renal excretion of salicylic acid becomes increasingly important as the metabolic pathways become saturated, because it is extremely sensitive to changes in urinary pH. A 10- to 20-fold increase in renal clearance occurs when urine pH is increased from 5 to 8. The use of urinary alkalinization exploits this particular aspect of salicylate elimination.[136]
History
Plant extracts, including willow bark and spiraea, of which salicylic acid was the active ingredient, had been known to help alleviate headaches, pains, and fevers since antiquity. The father of modern medicine, Hippocrates, who lived some time between 460 BC and 377 BC, left historical records describing the use of powder made from the bark and leaves of the willow tree to help these symptoms.[137]
A French chemist, Charles Frederic Gerhardt, was the first to prepare acetylsalicylic acid in 1853. In the course of his work on the synthesis and properties of various acid anhydrides, he mixed acetyl chloride with a sodium salt of salicylic acid (sodium salicylate). A vigorous reaction ensued, and the resulting melt soon solidified.[138] Since no structural theory existed at that time, Gerhardt called the compound he obtained “salicylic-acetic anhydride” (wasserfreie Salicylsäure-Essigsäure). This preparation of aspirin (“salicylic-acetic anhydride”) was one of the many reactions Gerhardt conducted for his paper on anhydrides and he did not pursue it further.
Six years later, in 1859, von Gilm obtained analytically pure acetylsalicylic acid (which he called acetylierte Salicylsäure, acetylated salicylic acid) by a reaction of salicylic acid and acetyl chloride.[139] In 1869, Schröder, Prinzhorn and Kraut repeated both Gerhardt’s (from sodium salicylate) and von Gilm’s (from salicylic acid) syntheses and concluded both reactions gave the same compound—acetylsalicylic acid. They were first to assign to it the correct structure with the acetyl group connected to the phenolic oxygen.[140]
In 1897, chemists working at Bayer AG produced a synthetically altered version of salicin, derived from the species Filipendula ulmaria (meadowsweet), which caused less digestive upset than pure salicylic acid. The identity of the lead chemist on this project is a matter of controversy. Bayer states the work was done by Felix Hoffmann, but the Jewish chemist Arthur Eichengrün later claimed he was the lead investigator and records of his contribution were expunged under the Nazi regime.[141][142] The new drug, formally acetylsalicylic acid, was named Aspirin by Bayer AG after the old botanical name for meadowsweet, Spiraea ulmaria. By 1899, Bayer was selling it around the world.[143] The name Aspirin is derived from “acetyl” and Spirsäure, an old German name for salicylic acid.[144] The popularity of aspirin grew over the first half of the 20th century, spurred by its supposed effectiveness in the wake of the Spanish flu pandemic of 1918. However, recent research suggests the high death toll of the 1918 flu was partly due to aspirin, as the doses used at times can lead to toxicity, fluid in the lungs, and, in some cases, contribute to secondary bacterial infections and mortality.[145] Aspirin’s profitability led to fierce competition and the proliferation of aspirin brands and products, especially after the American patent held by Bayer expired in 1917. [146][147]
The popularity of aspirin declined after the market releases of paracetamol (acetaminophen) in 1956 and ibuprofen in 1969.[148] In the 1960s and 1970s, John Vane and others discovered the basic mechanism of aspirin’s effects, while clinical trials and other studies from the 1960s to the 1980s established aspirin’s efficacy as an anticlotting agent that reduces the risk of clotting diseases.[149] Aspirin sales revived considerably in the last decades of the 20th century, and remain strong in the 21st century, because of its widespread use as a preventive treatment for heart attacks and strokes.[150]
Trademark
As part of war reparations specified in the 1919 Treaty of Versailles following Germany’s surrender after World War I, Aspirin (along with heroin) lost its status as a registered trademark in France, Russia, the United Kingdom, and the United States, where it became a generic name.[151][152][153] Today, aspirin is a generic word in Australia, France, India, Ireland, New Zealand, Pakistan, Jamaica, Colombia, the Philippines, South Africa, the United Kingdom and the United States.[154] Aspirin, with a capital “A”, remains a registered trademark of Bayer in Germany, Canada, Mexico, and in over 80 other countries, where the trademark is owned by Bayer, using acetylsalicylic acid in all markets, but using different packaging and physical aspects for each.[155][156]
Compendial status
Veterinary use
Aspirin is sometimes used for pain relief or as an anticoagulant in veterinary medicine, primarily in dogs and sometimes horses, although newer medications with fewer side effects are generally used, instead. Both dogs and horses are susceptible to the gastrointestinal side effects associated with salicylates, but it is a convenient treatment for arthritis in older dogs, and has shown some promise in cases of laminitis in horses.[159][160] Aspirin should be used in animals only under the direct supervision of a veterinarian; in particular, cats lack the glucuronide conjugates that aid in the excretion of aspirin, making even low doses potentially toxic.[161]
References
^ Sneader, W. (2000). “The discovery of aspirin: A reappraisal”. BMJ (Clinical research ed.) 321 (7276): 1591–1594. doi:10.1136/bmj.321.7276.1591. PMC 1119266. PMID 11124191.
^ Schrör, Karsten (2009). Acetylsalicylic acid. Wiley. ISBN 978-3-527-32109-4.
^ Paterson, John R.; Baxter, Gwendoline; Dreyer, Jacob S.; Halket, John M.; Flynn, Robert; Lawrence, James R. (2008). “Salicylic Acid sans Aspirin in Animals and Man: Persistence in Fasting and Biosynthesis from Benzoic Acid”. Journal of Agricultural and Food Chemistry 56 (24): 11648–11652. doi:10.1021/jf800974z. PMC 2800778. PMID 19053387.
^ Lewis, H. D.; Davis, J. W.; Archibald, D. G.; Steinke, W. E.; Smitherman, T. C.; Doherty Je, J. E.; Schnaper, H. W.; Lewinter, M. M.; Linares, E.; Pouget, J. M.; Sabharwal, S. C.; Chesler, E.; Demots, H. (1983). “Protective Effects of Aspirin against Acute Myocardial Infarction and Death in Men with Unstable Angina”. New England Journal of Medicine 309 (7): 396–403. doi:10.1056/NEJM198308183090703. PMID 6135989.
^ Julian, D G; D A Chamberlain, S J Pocock (24 September 1996). “A comparison of aspirin and anticoagulation following thrombolysis for myocardial infarction (the AFTER study): a multicentre unblinded randomised clinical trial”. BMJ (British Medical Journal) 313 (7070): 1429–1431. doi:10.1136/bmj.313.7070.1429. PMC 2353012. PMID 8973228.
^ Krumholz, H. M.; Radford, M. J.; Ellerbeck, E. F.; Hennen, J.; Meehan, T. P.; Petrillo, M.; Wang, Y.; Kresowik, T. F.; Jencks, S. F. (1995). “Aspirin in the treatment of acute myocardial infarction in elderly Medicare beneficiaries. Patterns of use and outcomes”. Circulation 92 (10): 2841–2847. doi:10.1161/01.CIR.92.10.2841. PMID 7586250.
^ Algra, Annemijn M; Rothwell, Peter M (2012). “Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials”. The Lancet Oncology 13 (5): 518–27. doi:10.1016/S1470-2045(12)70112-2. PMID 22440112.
^ Rothwell, Peter M; Price, Jacqueline F; Fowkes, F Gerald R; Zanchetti, Alberto; Roncaglioni, Maria Carla; Tognoni, Gianni; Lee, Robert; Belch, Jill FF et al. (2012). “Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: Analysis of the time course of risks and benefits in 51 randomised controlled trials”. The Lancet 379 (9826): 1602. doi:10.1016/S0140-6736(11)61720-0.|displayauthors=suggested (help)
^ Rothwell, Peter M; Wilson, Michelle; Price, Jacqueline F; Belch, Jill FF; Meade, Tom W; Mehta, Ziyah (2012). “Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials”. The Lancet 379 (9826): 1591. doi:10.1016/S0140-6736(12)60209-8.
^ a b c Macdonald S (2002). “Aspirin use to be banned in under 16-year olds”. BMJ 325 (7371): 988. doi:10.1136/bmj.325.7371.988/c. PMCÂ 1169585. PMIDÂ 12411346.
^ Burke, Anne; Smyth, Emer; FitzGerald, Garret A. (2006). “26: Analgesic Antipyretic and Antiinflammatory Agents”. Goodman and Gilman’s the pharmacological basis of therapeutics (11 ed.). New York: McGraw-Hill. pp. 671–716. ISBN 978-0-07-142280-2.
^ a b Warner, T. D.; Warner TD, Mitchell JA (2002). “Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum?”. Proc Natl Acad Sci USA 99 (21): 13371–3. PMC 129677. PMID 12374850.
^ “The use of aspirin”. Wordconstructions.com. Retrieved 11 May 2011.
^ “Aspirin”. The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
^ “Aspirin for Reducing Your Risk of Heart Attack and Stroke: Know the Facts”. U.S. Food and Drug Administration. Retrieved 26 July 2012.
^ “Aspirin for the Prevention of Cardiovascular Disease”. U.S. Preventive Services Task Force. Retrieved 26 July 2012.
^ Seshasai, SR; Wijesuriya, S; Sivakumaran, R; Nethercott, S; Erqou, S; Sattar, N; Ray, KK (13 February 2012). “Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials”. Archives of Internal Medicine 172 (3): 209–16. doi:10.1001/archinternmed.2011.628. PMID 22231610.
^ a b Algra, AM; Rothwell, PM (May 2012). “Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials”. The lancet oncology 13 (5): 518–27. doi:10.1016/S1470-2045(12)70112-2. PMID 22440112.
^ Sachs, C. J. (2005). “Oral analgesics for acute nonspecific pain”. American family physician 71 (5): 913–918. PMID 15768621.
^ Gaciong (2003). “The real dimension of analgesic activity of aspirin”. Thrombosis research 110 (5–6): 361–364. doi:10.1016/j.thromres.2003.08.009. PMID 14592563.
^ Derry, C. J.; Derry, S.; Moore, R. A. (2012). Caffeine as an analgesic adjuvant for acute pain in adults. In Derry, Sheena. “Cochrane Database of Systematic Reviews”. Cochrane database of systematic reviews (Online) 3: CD009281. doi:10.1002/14651858.CD009281.pub2. PMIDÂ 22419343.
^ “BLOWFISH (aspirin, caffeine) tablet, effervescent [Rally Labs LLC]“. DailyMed. U.S. Federal Drug Administration. Retrieved 27 July 2012.
^ Hersh, E.; Moore, P.; Ross, G. (2000). “Over-the-counter analgesics and antipyretics: A critical assessment”. Clinical Therapeutics 22 (5): 500–548. doi:10.1016/S0149-2918(00)80043-0. PMID 10868553.
^ Mett, A.; Tfelt-Hansen, P. (2008). “Acute migraine therapy: Recent evidence from randomized comparative trials”. Current Opinion in Neurology 21 (3): 331–337. doi:10.1097/WCO.0b013e3282fee843. PMID 18451718.
^ Kingery, WS (November 1997). “A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes”. Pain 73 (2): 123–39. doi:10.1016/S0304-3959(97)00049-3. PMID 9415498.
^ Loder, E; Rizzoli, P (12 January 2008). “Tension-type headache”. BMJ (Clinical research ed.) 336 (7635): 88–92. doi:10.1136/bmj.39412.705868.AD. PMC 2190284. PMID 18187725.
^ Gilmore, B; Michael, M (1 February 2011). “Treatment of acute migraine headache”. American family physician 83 (3): 271–80. PMID 21302868.
^ Bartfai, T; Conti, B (16 March 2010). “Fever”. TheScientificWorldJournal 10: 490–503. doi:10.1100/tsw.2010.50. PMC 2850202. PMID 20305990.
^ Pugliese, A; Beltramo, T; Torre, D (October 2008). “Reye’s and Reye’s-like syndromes”. Cell biochemistry and function 26 (7): 741–6. doi:10.1002/cbf.1465. PMID 18711704.
^ Beutler, AI; Chesnut, GT; Mattingly, JC; Jamieson, B (15 December 2009). “FPIN’s Clinical Inquiries. Aspirin use in children for fever or viral syndromes”. American family physician 80 (12): 1472. PMIDÂ 20000310.
^ “Medications Used to Treat Fever”. American Academy of Pediatrics. Retrieved 25 November 2012.
^ “51 FR 8180″. United States Federal Register 51 (45). 7 March 1986. Retrieved 25 November 2012.
^ Hall, SL; Lorenc, T (1 February 2010). “Secondary prevention of coronary artery disease”. American family physician 81 (3): 289–96. PMID 20112887.
^ Baigent C, Blackwell L, Collins R, Collins, R, Emberson, J, Godwin, J, Peto, R, Buring, J et al. (2009). “Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials”. Lancet 373 (9678): 1849–60. doi:10.1016/S0140-6736(09)60503-1. PMC 2715005. PMID 19482214.|displayauthors=suggested (help)
^ Wolff, T; Miller, T; Ko, S (17 March 2009). “Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force”. Annals of internal medicine 150 (6): 405–10. doi:10.7326/0003-4819-150-6-200903170-00009. PMID 19293073.
^ U.S. Preventive Services Task Force. “Aspirin for the Prevention of Cardiovascular Disease: Recommendation Statement”. Retrieved 15 August 2012.
^ Berger, JS; Lala, A, Krantz, MJ, Baker, GS, Hiatt, WR (July 2011). “Aspirin for the prevention of cardiovascular events in patients without clinical cardiovascular disease: a meta-analysis of randomized trials”. American heart journal 162 (1): 115–24.e2. doi:10.1016/j.ahj.2011.04.006. PMID 21742097.
^ Wang, TH; Bhatt, DL; Topol, EJ (March 2006). “Aspirin and clopidogrel resistance: an emerging clinical entity”. European heart journal 27 (6): 647–54. doi:10.1093/eurheartj/ehi684. PMID 16364973.
^ Oliveira, DC; Silva, RF; Silva, DJ; Lima, VC (September 2010). “Aspirin resistance: fact or fiction?”. Arquivos brasileiros de cardiologia 95 (3): e91–4. doi:10.1590/S0066-782X2010001300024. PMID 20944898.
^ Topçuoglu, MA; Arsava, EM; Ay, H (February 2011). “Antiplatelet resistance in stroke”. Expert Review of Neurotherapeutics 11 (2): 251–63. doi:10.1586/ern.10.203. PMC 3086673. PMID 21306212.
^ Ben-Dor, I; Kleiman, NS; Lev, E (15 July 2009). “Assessment, mechanisms, and clinical implication of variability in platelet response to aspirin and clopidogrel therapy”. The American journal of cardiology 104 (2): 227–33. doi:10.1016/j.amjcard.2009.03.022. PMID 19576352.
^ Norris, JW (September 2005). “Antiplatelet agents in secondary prevention of stroke: a perspective”. Stroke; a journal of cerebral circulation 36 (9): 2034–6. doi:10.1161/01.STR.0000177887.14339.46. PMID 16100022.
^ Sleight, P; Pouleur, H; Zannad, F (July 2006). “Benefits, challenges, and registerability of the polypill”. European heart journal 27 (14): 1651–6. doi:10.1093/eurheartj/ehi841. PMID 16603580.
^ National Guideline Clearinghouse (NGC). “2011 ACCF/AHA/SCAI guideline for percutaneous coronary artery intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions.”. United States Agency for Healthcare Research and Quality (AHRQ). Retrieved 28 August 2012.
^ Musumeci, G; Di Lorenzo, E; Valgimigli, M (December 2011). “Dual antiplatelet therapy duration: what are the drivers?”. Current opinion in cardiology. 26 Suppl 1: S4–14. doi:10.1097/01.hco.0000409959.11246.ba. PMID 22129582.
^ Manzano, A; Pérez-Segura, P (2012). “Colorectal cancer chemoprevention: is this the future of colorectal cancer prevention?”. TheScientificWorldJournal 2012: 327341. doi:10.1100/2012/327341. PMC 3353298. PMID 22649288.
^ Chan, AT; Arber, N; Burn, J; Chia, WK; Elwood, P; Hull, MA; Logan, RF; Rothwell, PM; Schrör, K; Baron, JA (February 2012). “Aspirin in the chemoprevention of colorectal neoplasia: an overview”. Cancer prevention research (Philadelphia, Pa.) 5 (2): 164–78. doi:10.1158/1940-6207.CAPR-11-0391. PMC 3273592. PMID 22084361.
^ Thun, MJ; Jacobs, EJ; Patrono, C (3 April 2012). “The role of aspirin in cancer prevention”. Nature reviews. Clinical oncology 9 (5): 259–67. doi:10.1038/nrclinonc.2011.199. PMID 22473097.
^ Rothwell, PM; Wilson, M; Elwin, CE; Norrving, B; Algra, A; Warlow, CP; Meade, TW (20 November 2010). “Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials”. Lancet 376 (9754): 1741–50. doi:10.1016/S0140-6736(10)61543-7. PMID 20970847.
^ U.S. Preventive Services Task, Force (6 March 2007). “Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement”. Annals of internal medicine 146 (5): 361–4. doi:10.7326/0003-4819-146-5-200703060-00008. PMID 17339621.
^ a b National Heart Foundation of Australia (RF/RHD guideline development working group) and the Cardiac Society of Australia and New Zealand (2006). “Diagnosis and management of acute rheumatic fever and rheumatic heart disease in Australia. An evidence-based review” (PDF). National Heart Foundation of Australia. pp. 33–37. Retrieved 9 May 2011.
^ Working Group on Pediatric Acute Rheumatic Fever and Cardiology Chapter of Indian Academy of, Pediatrics; Saxena, A; Kumar, RK; Gera, RP; Radhakrishnan, S; Mishra, S; Ahmed, Z (July 2008). “Consensus guidelines on pediatric acute rheumatic fever and rheumatic heart disease”. Indian pediatrics 45 (7): 565–73. PMID 18695275.
^ Hashkes; Tauber, T.; Somekh, E.; Brik, R.; Barash, J.; Mukamel, M.; Harel, L.; Lorber, A.; Berkovitch, M.; Uziel, Y.; Pediatric Rheumatlogy Study Group of Israel (2003). “Naproxen as an alternative to aspirin for the treatment of arthritis of rheumatic fever: a randomized trial”. The Journal of pediatrics 143 (3): 399–401. doi:10.1067/S0022-3476(03)00388-3. PMID 14517527.
^ Rowley, AH; Shulman, ST (February 2010). “Pathogenesis and management of Kawasaki disease”. Expert review of anti-infective therapy 8 (2): 197–203. doi:10.1586/eri.09.109. PMC 2845298. PMID 20109049.
^ Baumer, JH; Love, SJ; Gupta, A; Haines, LC; Maconochie, I; Dua, JS (18 October 2006). “Salicylate for the treatment of Kawasaki disease in children”. In Baumer, J Harry. Cochrane database of systematic reviews (Online) (4): CD004175. doi:10.1002/14651858.CD004175.pub2. PMIDÂ 17054199.
^ Duley, L.; Henderson-Smart, D. J.; Meher, S.; King, J. F. (2007). Antiplatelet agents for preventing pre-eclampsia and its complications. In Duley, Lelia. “Cochrane Database of Systematic Reviews”. Cochrane database of systematic reviews (Online) (2): CD004659. doi:10.1002/14651858.CD004659.pub2. PMIDÂ 17443552.
^ Roberge, S. P.; Villa, P.; Nicolaides, K.; Giguère, Y.; Vainio, M.; Bakthi, A.; Ebrashy, A.; Bujold, E. (2012). “Early Administration of Low-Dose Aspirin for the Prevention of Preterm and Term Preeclampsia: A Systematic Review and Meta-Analysis”. Fetal Diagnosis and Therapy 31 (3): 141–146. doi:10.1159/000336662. PMID 22441437.
^ Dorsch MP, Lee JS, Lynch DR, Dunn SP, Rodgers JE, Schwartz T, Colby E, Montague D, Smyth SS (2007). “Aspirin Resistance in Patients with Stable Coronary Artery Disease with and without a History of Myocardial Infarction”. Ann Pharmacother 41 (May): 737–41. doi:10.1345/aph.1H621. PMID 17456544.
^ Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR (2008). “Aspirin “resistance” and risk of cardiovascular morbidity: systematic review and meta-analysis”. BMJ 336 (7637): 195–8. doi:10.1136/bmj.39430.529549.BE. PMC 2213873. PMID 18202034.
^ Pignatelli P, Di Santo S, Barillà F, Gaudio C, Violi F (2008). “Multiple anti-atherosclerotic treatments impair aspirin compliance: effects on aspirin resistance”. J. Thromb. Haemost. 6 (10): 1832–4. doi:10.1111/j.1538-7836.2008.03122.x. PMID 18680540.
^ Tilo Grosser, Susanne Fries, John A. Lawson, Shiv C. Kapoor, Gregory R. Grant and Garret A. FitzGerald (2013). “Drug Resistance and Pseudoresistance: An Unintended Consequence of Enteric Coating Aspirin”. Circulation (4 December 2012) 127 (3): 377–85. doi:10.1161/CIRCULATIONAHA.112.117283. PMC 3552520. PMID 23212718. Retrieved 5 December 2012. Lay summary – The New York Times (4 December 2012).
^ a b British National Formulary (45 ed.). British Medical Journal and Royal Pharmaceutical Society of Great Britain. 2003.
^ “Aspirin monograph: dosages, etc”. Medscape.com. Retrieved 11 May 2011.
^ (USPSTF, March 2009)
^ a b c “Aspirin: More Evidence That Low Dose Is All That Is Needed (from Medscape)”. Cme.medscape.com. Retrieved 11 May 2011.
^ British National Formulary for Children. British Medical Journal and Royal Pharmaceutical Society. 2006.
^ a b c d e “Aspirin information from Drugs.com”. Drugs.com. Archived from the original on 9 May 2008. Retrieved 8 May 2008.
^ a b c “Oral Aspirin information”. First DataBank. Archived from the original on 9 June 2008. Retrieved 8 May 2008.
^ Raithel M; Baenkler HW; Naegel A; Buchwald, F; Schultis, HW; Backhaus, B; Kimpel, S; Koch, H et al. (2005). “Significance of salicylate intolerance in diseases of the lower gastrointestinal tract” (PDF). J. Physiol. Pharmacol. 56 Suppl 5: 89–102. PMID 16247191.|displayauthors=suggested (help)
^ Senna GE, Andri G, Dama AR, Mezzelani P, Andri L (1995). “Tolerability of imidazole salycilate in aspirin-sensitive patients”. Allergy Proc 16 (5): 251–4. doi:10.2500/108854195778702675. PMID 8566739.
^ a b “PDR Guide to Over the Counter (OTC) Drugs”. Archived from the original on 10 April 2008. Retrieved 28 April 2008.
^ Livingstone, Frank B. (1985). Frequencies of hemoglobin variants: thalassemia, the glucose-6-phosphate dehydrogenase deficiency, G6PD variants, and ovalocytosis in human populations. Oxford University Press. ISBNÂ 0-19-503634-4.
^ “Dengue and Dengue Hemorrhagic Fever: Information for Health Care Practitioners”. Archived from the original on 17 March 2008. Retrieved 28 April 2008.
^ a b c Sørensen HT; Mellemkjaer L; Blot WJ; Nielsen, Gunnar Lauge; Steffensen, Flemming Hald; McLaughlin, Joseph K.; Olsen, Jorgen H. (2000). “Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin”. Am. J. Gastroenterol. 95 (9): 2218–24. doi:10.1111/j.1572-0241.2000.02248.x. PMID 11007221.
^ Delaney JA, Opatrny L, Brophy JM & Suissa S (2007). “Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding”. CMAJ 177 (4): 347–51. doi:10.1503/cmaj.070186. PMC 1942107. PMID 17698822.
^ Wallace, J. L. (2008). “Prostaglandins, NSAIDs, and Gastric Mucosal Protection: Why Doesn’t the Stomach Digest Itself?”. Physiological Reviews 88 (4): 1547–1565. doi:10.1152/physrev.00004.2008. PMID 18923189.
^ Fiorucci, S.; Santucci, L.; Wallace, J. L.; Sardina, M.; Romano, M.; Del Soldato, P.; Morelli, A. (2003). “Interaction of a selective cyclooxygenase-2 inhibitor with aspirin and NO-releasing aspirin in the human gastric mucosa”. Proceedings of the National Academy of Sciences 100 (19): 10937–10941. doi:10.1073/pnas.1933204100. PMC 196906. PMID 12960371.
^ “General Chemistry Online: FAQ: Acids and bases: What is the buffer system in buffered aspirin?”. Antoine.frostburg.edu. Retrieved 11 May 2011.
^ Dammann, H. G.; Saleki, M.; Torz, M.; Schulz, H. U.; Krupp, S.; Schürer, M.; Timm, J.; Gessner, U. (2004). “Effects of buffered and plain acetylsalicylic acid formulations with and without ascorbic acid on gastric mucosa in healthy subjects”. Alimentary pharmacology & therapeutics 19 (3): 367–374. doi:10.1111/j.1365-2036.2004.01742.x. PMID 14984384.
^ Konturek; Kania, J; Hahn, EG; Konturek, JW (2006). “Ascorbic acid attenuates aspirin-induced gastric damage: role of inducible nitric oxide synthase”. J Physiol Pharmacol. 57 Suppl 5 (5): 125–36. PMID 17218764.
^ Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL (2003). “Salicylate induces tinnitus through activation of cochlear NMDA receptors”. J. Neurosci. 23 (9): 3944–52. PMID 12736364.
^ a b Belay ED, Bresee JS, Holman RC, Khan AS, Shahriari A, Schonberger LB (1999). “Reye’s syndrome in the United States from 1981 through 1997″. N. Engl. J. Med. 340 (18): 1377–82. doi:10.1056/NEJM199905063401801. PMID 10228187.
^ Reye’s syndrome. nhs.uk
^ Berges-Gimeno MP & Stevenson DD (2004). “Nonsteroidal anti-inflammatory drug-induced reactions and desensitization”. J Asthma 41 (4): 375–84. doi:10.1081/JAS-120037650. PMID 15281324.
^ Vernooij MW, Haag MD, der Lugt A, Hofman A, Krestin GP, Stricker BH, Breteler MM (2009). “Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study”. Arch Neurol 66 (6): 714–20. doi:10.1001/archneurol.2009.42. PMID 19364926.
^ Gorelick PB (2009). “Cerebral microbleeds: evidence of heightened risk associated with aspirin use”. Arch Neurol 66 (6): 691–3. doi:10.1001/archneurol.2009.85. PMID 19506128.
^ a b He, J.; Whelton, P. K.; Vu, B.; Klag, M. J. (1998). “Aspirin and risk of hemorrhagic stroke: A meta-analysis of randomized controlled trials”. JAMA: the Journal of the American Medical Association 280 (22): 1930–1935. PMID 9851479.
^ Saloheimo, P.; Ahonen, M.; Juvela, S.; Pyhtinen, J.; Savolainen, E. R.; Hillbom, M. (2005). “Regular Aspirin-Use Preceding the Onset of Primary Intracerebral Hemorrhage is an Independent Predictor for Death”. Stroke 37 (1): 129–133. doi:10.1161/01.STR.0000196991.03618.31. PMID 16322483.
^ Medical knowledge self-assessment program for students 4, By American College of Physicians, Clerkship Directors in Internal Medicine, Nephrology 227, Item 29
^ Scher, K.S. (1996). “Unplanned reoperation for bleeding”. Am Surg 62 (1): 52–55. PMID 8540646.
^ Kreplick, LW. (2001). “Salicylate Toxicity in Emergency Medicine”. Medscape.
^ Gaudreault P, Temple AR, Lovejoy FH Jr (1982). “The relative severity of acute versus chronic salicylate poisoning in children: a clinical comparison”. Pediatrics 70 (4): 566–9. PMID 7122154. (primary source)
^ Marx, John (2006). Rosen’s emergency medicine: concepts and clinical practice. Mosby/Elsevier. p. 2242. ISBN 978-0-323-02845-5.
^ Morra P, Bartle WR, Walker SE, Lee SN, Bowles SK, Reeves RA (1996). “Serum concentrations of salicylic acid following topically applied salicylate derivatives”. Ann. Pharmacother 30 (9): 935–40. PMID 8876850.
^ R. Baselt (2011). Disposition of Toxic Drugs and Chemicals in Man (9th ed.). Seal Beach, California: Biomedical Publications. pp. 20–23.
^ “Information for Healthcare Professionals: Concomitant Use of Ibuprofen and Aspirin”. FDA. U.S. Department of Health & Human Services. 2006-09. Archived from the original on 13 November 2010. Retrieved 22 November 2010.
^ Katzung (1998), Basic and Clinical Pharmacology, McGraw-Hill, p. 584.
^ Loh HS, Watters K & Wilson CW (1 November 1973). “The Effects of Aspirin on the Metabolic Availability of Ascorbic Acid in Human Beings”. J Clin Pharmacol 13 (11): 480–6. PMID 4490672.
^ Basu TK (1982). “Vitamin C-aspirin interactions”. Int J Vitam Nutr Res Suppl 23: 83–90. PMID 6811490.
^ Ioannides C, Stone AN, Breacker PJ & Basu TK (1982). “Impairment of absorption of ascorbic acid following ingestion of aspirin in guinea pigs”. Biochem Pharmacol 31 (24): 4035–8. doi:10.1016/0006-2952(82)90652-9. PMID 6818974.
^ EF Reynolds, ed. (1982). “Aspirin and similar analgesic and anti-inflammatory agents”. Martindale: The Extra Pharmacopoeia (28th ed.). pp. 234–82.
^ Richard Leroy Myers (30 August 2007). The 100 Most Important Chemical Compounds: A Reference Guide. ABC-CLIO. p. 10. ISBN 978-0-313-33758-1. Retrieved 18 November 2012.
^ Palleros, Daniel R. (2000). Experimental Organic Chemistry. New York: John Wiley & Sons. p. 494. ISBN 0-471-28250-2.
^ Barrans, Richard. “Aspirin Aging”. Newton BBS. Archived from the original on 18 May 2008. Retrieved 8 May 2008.
^ Carstensen, J.T.; F Attarchi and XP Hou (1985). “Decomposition of aspirin in the solid state in the presence of limited amounts of moisture”. Journal of Pharmaceutical Sciences 77 (4): 318–21. doi:10.1002/jps.2600770407. PMID 4032246.
^ “Acetylsalicylic acid”. Jinno Laboratory, School of Materials Science, Toyohashi University of Technology. 4 March 1996. Retrieved 7 September 2007.
^ Vishweshwar, P.; McMahon, J. A.; Oliveira, M.; Peterson, M. L.; Zaworotko, M. J. (2005). “The Predictably Elusive Form II of Aspirin”. Journal of the American Chemical Society 127 (48): 16802–16803. doi:10.1021/ja056455b. PMID 16316223.
^ Bond, Andrew D.; Boese, Roland; and Desiraju, Gautam R. (2007). “On the Polymorphism of Aspirin: Crystalline Aspirin as Intergrowths of Two “Polymorphic” Domains”. Angewandte Chemie International Edition 46 (4): 618–622. doi:10.1002/anie.200603373. PMID 17139692.
^ Vane, John Robert (1971). “Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs”. Nature – New Biology 231 (25): 232–5. PMID 5284360.
^ Vane JR, Botting RM (2003). “The mechanism of action of aspirin” (PDF). Thromb Res 110 (5–6): 255–8. doi:10.1016/S0049-3848(03)00379-7. PMID 14592543.
^ “The Nobel Prize in Physiology or Medicine 1982″. Nobelprize.or.
^ “Aspirin in Heart Attack and Stroke Prevention”. American Heart Association. Archived from the original on 31 March 2008. Retrieved 8 May 2008.
^ Tohgi, H; S Konno, K Tamura, B Kimura and K Kawano (1992). “Effects of low-to-high doses of aspirin on platelet aggregability and metabolites of thromboxane A2 and prostacyclin”. Stroke 23 (10): 1400–1403. doi:10.1161/01.STR.23.10.1400. PMID 1412574.
^ Achhrish goel, Ruchi gupta Anubhav goswami Madhu soodan sharma Yogesh sharma (2011). Pharmacokinetic Solubility And Dissolution Profile of Non-Steroidal Anti-Inflammatory Drugs 2 (3).
^ MartÃnez-González J, Badimon L (2007). “Mechanisms underlying the cardiovascular effects of COX-inhibition: benefits and risks”. Curr Pharm Des 13 (22): 2215–27. doi:10.2174/138161207781368774. PMID 17691994.
^ Funk CD, FitzGerald GA (November 2007). “COX-2 inhibitors and cardiovascular risk”. J Cardiovasc Pharmacol 50 (5): 470–9. doi:10.1097/FJC.0b013e318157f72d. PMID 18030055.
^ Somasundaram; Sigthorsson, G; Simpson, RJ; Watts, J; Jacob, M; Tavares, IA; Rafi, S; Roseth, A et al. (2000). “Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat”. Aliment Pharmacol Ther 14 (5): 639–650. doi:10.1046/j.1365-2036.2000.00723.x. PMID 10792129.|displayauthors=suggested (help)
^ Paul-Clark, Mark J.; Cao, Thong van; Moradi-Bidhendi, Niloufar; Cooper, Dianne & Gilroy, Derek W. (2004). “15-epi-lipoxin A4–mediated Induction of Nitric Oxide Explains How Aspirin Inhibits Acute Inflammation”. J. Exp. Med. 200 (1): 69–78. doi:10.1084/jem.20040566. PMC 2213311. PMID 15238606.
^ McCarty, M. F.; Block, K. I. (2006). “Preadministration of high-dose salicylates, suppressors of NF-kappaB activation, may increase the chemosensitivity of many cancers: an example of proapoptotic signal modulation therapy”. Integr Cancer Ther. 5 (3): 252–268. doi:10.1177/1534735406291499. PMID 16880431.
^ Hawley, S. A.; Fullerton, M. D.; Ross, F. A.; Schertzer, J. D.; Chevtzoff, C.; Walker, K. J.; Peggie, M. W.; Zibrova, D.; Green, K. A.; Mustard, K. J.; Kemp, B. E.; Sakamoto, K.; Steinberg, G. R.; Hardie, D. G. (2012). “The Ancient Drug Salicylate Directly Activates AMP-Activated Protein Kinase”. Science 336 (6083): 918–922. doi:10.1126/science.1215327. PMC 3399766. PMID 22517326.
^ Raffensperger, Lisa (19 April 2012). “Clues to aspirin’s anti-cancer effects revealed”. New Scientist 214 (2862): 16. doi:10.1016/S0262-4079(12)61073-2.
^ Bhat (2009). “Aspirin inhibits camptothecin-induced p21CIP1 levels and potentiates apoptosis in human breast cancer cells”. International Journal of Oncology 34 (3). doi:10.3892/ijo_00000185.
^ Bhat (2009). “Does aspirin acetylate multiple cellular proteins? (Review)”. Molecular Medicine Reports 2 (4). doi:10.3892/mmr_00000132.
^ Caviezel, F.; Cattaneo, A. G.; Tell, A.; Corino, T.; Mascherpa, M. (1983). “The effect of acetylsalicylic acid and diclofenac on stimulated growth hormone and prolactin secretion in humans”. International journal of clinical pharmacology, therapy, and toxicology 21 (10): 502–504. PMID 6642786.
^ Ramey, JN; Burrow, GN; Spaulding, SW; Donabedian, RK; Speroff, L; Frantz, AG (1976). “The effect of aspirin and indomethacin on the TRH response in man”. The Journal of Clinical Endocrinology and Metabolism 43 (1): 107–14. doi:10.1210/jcem-43-1-107. PMID 820703.
^ a b Nye EJ, Hockings GI, Grice JE, Torpy DJ, Walters MM, Crosbie GV, Wagenaar M, Cooper M, Jackson RV (1997). “Aspirin inhibits vasopressin-induced hypothalamic-pituitary-adrenal activity in normal humans”. J. Clin. Endocrinol. Metab. 82 (3): 812–7. doi:10.1210/jc.82.3.812. PMID 9062488.
^ Hockings GI, Grice JE, Crosbie GV, Walters MM, Jackson AJ, Jackson RV (1993). “Aspirin increases the human hypothalamic-pituitary-adrenal axis response to naloxone stimulation”. J. Clin. Endocrinol. Metab. 77 (2): 404–8. doi:10.1210/jc.77.2.404. PMID 8393884.
^ Ferguson, RK; Boutros, AR (17 August 1970). “Death following self-poisoning with aspirin”. Journal of the American Medical Association 213 (7): 1186–8. doi:10.1001/jama.213.7.1186. PMID 5468267.
^ Kaufman, FL; Dubansky, AS (1970-04). “Darvon poisoning with delayed salicylism: a case report”. Pediatrics 49 (4): 610–1. PMID 5013423.
^ a b c Levy, G; Tsuchiya, T (31 September 1972). “Salicylate accumulation kinetics in man”. New England Journal of Medicine 287 (9): 430–2. doi:10.1056/NEJM197208312870903. PMID 5044917.
^ 2,3-Dihydroxybenzoic acid is a product of human aspirin metabolism. Martin Grootveld and Barry Halliwell, Biochemical Pharmacology, Volume 37, Issue 2, 15 January 1988, pages 271–280, doi:10.1016/0006-2952(88)90729-0
^ Hartwig, Otto H (14 November 1983). “Pharmacokinetic considerations of common analgesics and antipyretics”. American Journal of Medicine 75 (5A): 30–7. doi:10.1016/0002-9343(83)90230-9. PMID 6606362.
^ Done, AK (1960-11). “Salicylate intoxication. Significance of measurements of salicylate in blood in cases of acute ingestion”. Pediatrics 26: 800–7. PMID 13723722.
^ Chyka PA, Erdman AR, Christianson G, Wax PM, Booze LL, Manoguerra AS, Caravati EM, Nelson LS, Olson KR, Cobaugh DJ, Scharman EJ, Woolf AD, Troutman WG; Americal Association of Poison Control Centers; Healthcare Systems Bureau, Health Resources and Services Administration, Department of Health and Human Services. (2007). “Salicylate poisoning: an evidence-based consensus guideline for out-of-hospital management”. Clin Toxicol (Phila) 45 (2): 95–131. doi:10.1080/15563650600907140. PMID 17364628.
^ Prescott LF, Balali-Mood M, Critchley JA, Johnstone AF, Proudfoot AT (1982). “Diuresis or urinary alkalinisation for salicylate poisoning?”. Br Med J (Clin Res Ed) 285 (6352): 1383–6. doi:10.1136/bmj.285.6352.1383. PMC 1500395. PMID 6291695.
^ Dargan PI, Wallace CI, Jones AL. (2002). “An evidenced based flowchart to guide the management of acute salicylate (aspirin) overdose”. Emerg Med J 19 (3): 206–9. doi:10.1136/emj.19.3.206. PMC 1725844. PMID 11971828.
^ Mary Bellis (16 June 2010). “History of aspirin”. Inventors.about.com. Retrieved 11 May 2011.
^ (German) Gerhardt C (1853). “Untersuchungen über die wasserfreien organischen Säuren”. Annalen der Chemie und Pharmacie 87: 149–179. doi:10.1002/jlac.18530870107.
^ (German) von Gilm H (1859). “Acetylderivate der Phloretin- und Salicylsäure”. Annalen der Chemie und Pharmacie 112 (2): 180–185. doi:10.1002/jlac.18591120207.
^ (German)Schröder, Prinzhorn, Kraut K (1869). “Uber Salicylverbindungen”. Annalen der Chemie und Pharmacie 150 (1): 1–20. doi:10.1002/jlac.18691500102.
^ Mahdi, JG; Mahdi, AJ, Mahdi, AJ, Bowen, ID (April 2006). “The historical analysis of aspirin discovery, its relation to the willow tree and antiproliferative and anticancer potential”. Cell proliferation 39 (2): 147–55. doi:10.1111/j.1365-2184.2006.00377.x. PMID 16542349.
^ Sneader W (23–30 December 2000). “The discovery of aspirin: a reappraisal”. BMJ 321 (7276): 1591–4. doi:10.1136/bmj.321.7276.1591. PMC 1119266. PMID 11124191.
^ Jeffreys 2005, pp. 73
^ Singer, H. (1901). “Ueber Aspirin”. Pflügers Archiv: European Journal of Physiology 84 (11–12): 527–546. doi:10.1007/BF01769129.
^ Starko, Karen M. (2009). “Salicylates and Pandemic Influenza Mortality, 1918–1919 Pharmacology, Pathology, and Historic Evidence”. Clinical Infectious Diseases 49 (9): 1405. doi:10.1086/606060.
^ Jeffreys 2005, pp. 136–142, 151–152
^ “Bayer patents aspirin – This Day in History – 3/6/1899″. History.com. Retrieved 11 May 2011.
^ Jeffreys 2005, pp. 212–217
^ Jeffreys 2005, pp. 226–231
^ Jeffreys 2005, pp. 267–269
^ “Treaty of Versailles, Part X, Section IV, Article 298″. 28 June 1919. pp. Annex, Paragraph 5. Retrieved 25 October 2008.
^ Mehta, Aalok (2005). “Aspirin”. Chemical & Engineering News 83 (25). Retrieved 23 October 2008.
^ “The Centenary of Aspirin”. Ul.ie. 6 March 1999. Retrieved 11 May 2011.
^ CBE Style Manual Committee; Huth, Edward J. (1994). Scientific Style and Format: The CBE Manual for Authors, Editors, and Publishers. Cambridge University Press. p. 164. ISBN 978-0-521-47154-1.
^ “Aspirin: the versatile drug”. CBC News. 28 May 2009.
^ Cheng, Tsung O. (2007). “The History of Aspirin”. Texas Heart Institute Journal 34 (3): 392–393. PMC 1995051. PMID 17948100.
^ Sigma Aldrich. “Aspirin”. Retrieved 13 July 2009.
^ British Pharmacopoeia. “Index BP 2009″. Retrieved 13 July 2009.
^ Crosby, Janet Tobiassen (2006). “Veterinary Questions and Answers”. About.com. Archived from the original on 8 September 2007. Retrieved 5 September 2007.
^ Cambridge H, Lees P, Hooke RE, Russell CS (1991). “Antithrombotic actions of aspirin in the horse”. Equine Vet J 23 (2): 123–7. doi:10.1111/j.2042-3306.1991.tb02736.x. PMID 1904347.
^ Lappin, Michael R., ed. (2001). Feline internal medicine secrets. Philadelphia: Hanley & Belfus. p. 160. ISBN 1-56053-461-3.
Bibliography
Jeffreys, Diarmuid (11 August 2005). Aspirin: The Remarkable Story of a Wonder Drug. Bloomsbury USA. ISBNÂ 1-58234-600-3.
External links
- NextBio Aspirin Entry
Aspirin bound to proteins in the PDB- The History of Aspirin
Aspirin at The Periodic Table of Videos (University of Nottingham)- How Aspirin works
- The science behind aspirin
Take two: Aspirin, New uses and new dangers are still being discovered as aspirin enters its 2nd century. Shauna Roberts, American Chemical Society
Ling, Greg (2005). “Aspirin”. How Products are Made 1. Thomson Gale.- U.S. National Library of Medicine: Drug Information Portal – Aspirin
- CDC – NIOSH Pocket Guide to Chemical Hazards – Acetylsalicylic Acid




No comments:
Post a Comment